Research
Wound healing is one of the most complex processes our bodies must perform. Following damage to our skin, bones, nerves, heart, and other tissues, a variety of cells work together over time to repair the damage. However, in many cases, this process of repair does not work out as well as we would like – our skin may fail to repair itself and close our bodies off to the outside world and the bacteria and viruses that live there (e.g. diabetic foot ulcers, venous ulcers), bones may fail to stitch themselves back together (e.g. non-union tibia fractures), or large traumatic wounds may result in excessive scarring that limits mobility, the ability to regulate body temperature, and the ability to feel touch (e.g. large area burns).
In many cases, materials provide an attractive option for helping our bodies heal damage. Unfortunately, most materials are either inactive bystanders during wound healing or provide limited therapeutic influence that is pre-programmed into the material, not responsive to the changing conditions of the healing wound over time. Therefore, there is a significant opportunity to develop new methods to create dynamic biomaterials that constructively interact with our healing bodies over time to promote improved outcomes (e.g. faster healing, less scarring).
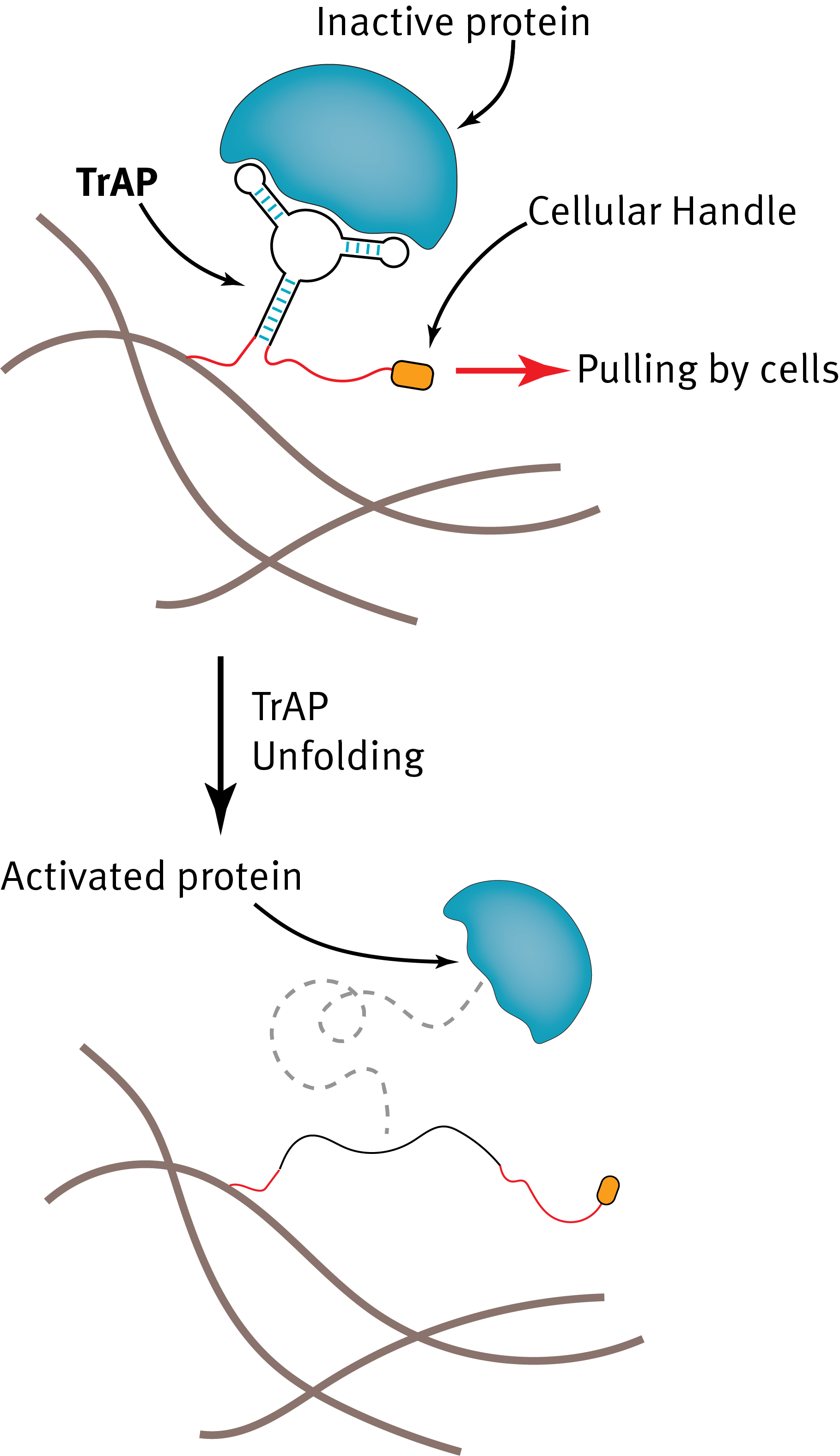
Our lab is pioneering the use of nanotechnology-based approaches for designing dynamic biomaterials that work together with our bodies to promote healing. Recently, we have developed TrAPs (Traction-Force Activated Payloads) - a bioinspired nanotechnology that enables cells to pull on materials to activate specific signals for different types of cells that, when combined, help to heal the damage. We are fascinated by how this new approach to designing cell-responsive materials will enable potentially revolutionary methods for actively guiding the healing of tissues towards a more regenerative outcome.
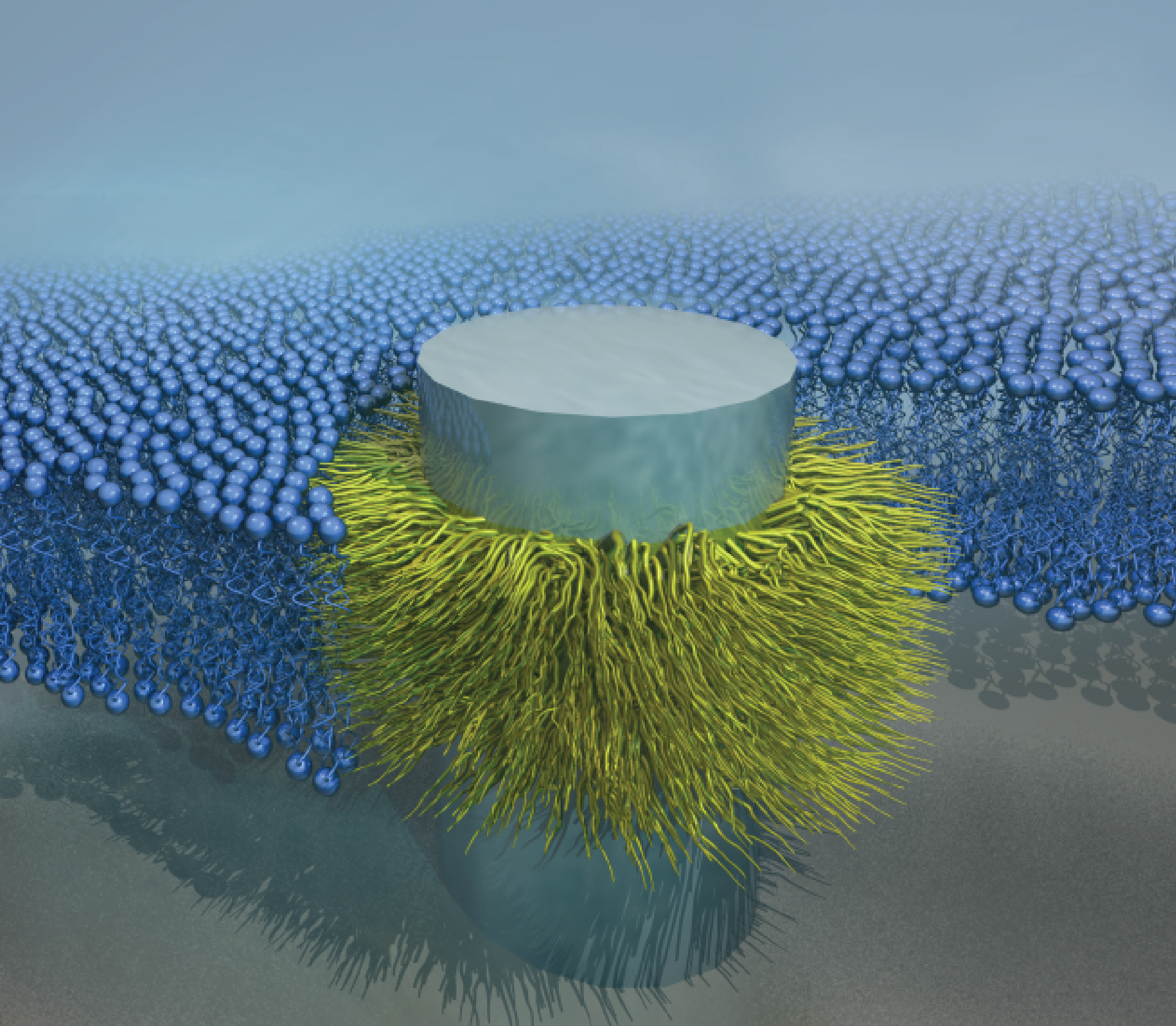
Advances in nanofabrication have enabled the ability to create devices that replicate the natural length scales of biological systems. This ability to engineer nanostructured devices is providing new opportunities for seamlessly merging biological systems such as cells (e.g. neurons) with inorganic devices (e.g. computer chips). In doing so, it will be possible to unlock unprecedented ability to seamlessly bridge the natural and engineered worlds and usher in a new understanding of areas ranging from neural communication to the harvesting of bioelectricity.
We are interested in using bioinspiration to design new methods for seamlessly bridging the biotic-abiotic interface. In the past, this strategy has been used to create nanoscale silicon probes that seamlessly fuse into cellular membranes. Using similar approaches to bioinspired design, we are working to develop new devices that will allow us to begin deciphering long-standing biological questions.